Atomic Number 3
Atomic Number 38
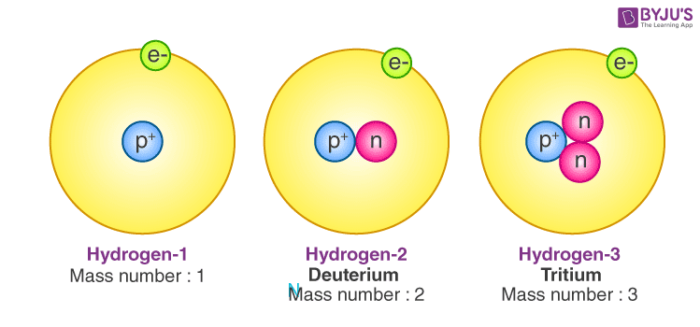
'stone') is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the lightest metal and the lightest solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in mineral oil. If you haven't solved the crossword clue Atomic number 3 yet try to search our Crossword Dictionary by entering the letters you already know! (Enter a dot for each missing letters, e.g. “P.ZZ.” will find “PUZZLE”.) Also look at the related clues for crossword clues with similar answers to “Atomic number 3” Contribute to Crossword Clues.
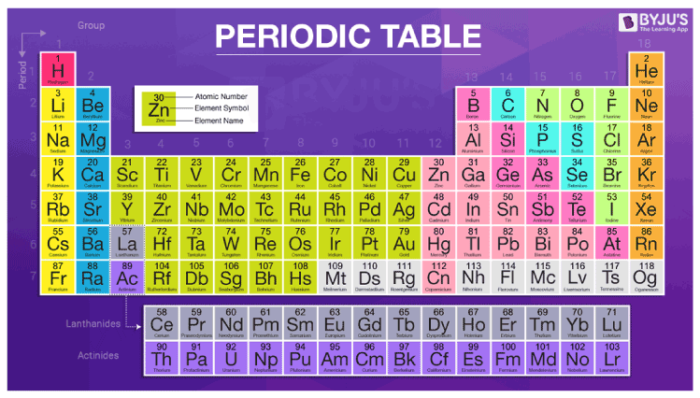
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behavior of an element.
Element With 3 Atomic Number
In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column (group). For instance, all of the elements in Group 1A are relatively soft metals, react violently with water, and form 1+ charges; all of the elements in Group 8A are unreactive, monatomic gases at room temperature, etc. In other words, there is a periodic repetition of the properties of the chemical elements with increasing mass.
Atomic #37 Crossword Clue
Lithium Ion Atomic Number
In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass— at that time, the nucleus had not yet been discovered, and there was no understanding at all of the interior structure of the atom, so atomic mass was the only guide to use. Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed the properties of the elements.